The team was created in 2007 by Pr Yves Lévy and is studying HIV pathophysiology from upstream to translational research in the field of immune interventions and vaccines. The research activity of the team is closely linked to the department of clinical immunopathology. This department has been involved in more than 20 clinical trials in the field of HIV and primary immunodeficiencies, and more than 1000 HIV-infected patients are followed in this clinical department. In 2007, the clinical department extended its clinical research program by the creation of a Clinical Center of HIV Vaccinology aiming at recruiting healthy volunteers and developing an HIV-vaccine clinical trials program. Therefore, extensive clinical opportunities have been available, allowing thus the group to extensively publish on the topic of Immune-Based Therapies and HIV physiopathology.
 Dendritic cells matured ex vivo in presence of HIV lipopeptides (© Institut Pasteur, VRI)
Dendritic cells matured ex vivo in presence of HIV lipopeptides (© Institut Pasteur, VRI)
More recently, in the context of the HIV vaccine program of the ANRS (French National Agency of Research on HIV and hepatitis), the team 16 has extended its technology capacity and expertise by the creation of a platform (Mondor Immunology Center; M.I.C) to perform key end-point immune assays aimed to evaluate human immune responses in immunotherapeutic and vaccine clinical trials. In 2011, a new step of the ANRS vaccine program was achieved by the creation of the Vaccine Research Institute (VRI), lauréat A+ Labex “investissement d’avenir”. Y. Lévy is the PI and coordinator of the VRI on behalf of the ANRS and UPEC. The VRI is a labex located in Mondor with inside and outside teams aimed to face the challenges to develop effective vaccines against HIV and HCV. This research program is implemented by a large network of key international opinion leaders in this field through a unique collaborative network and with a central strategic plan.
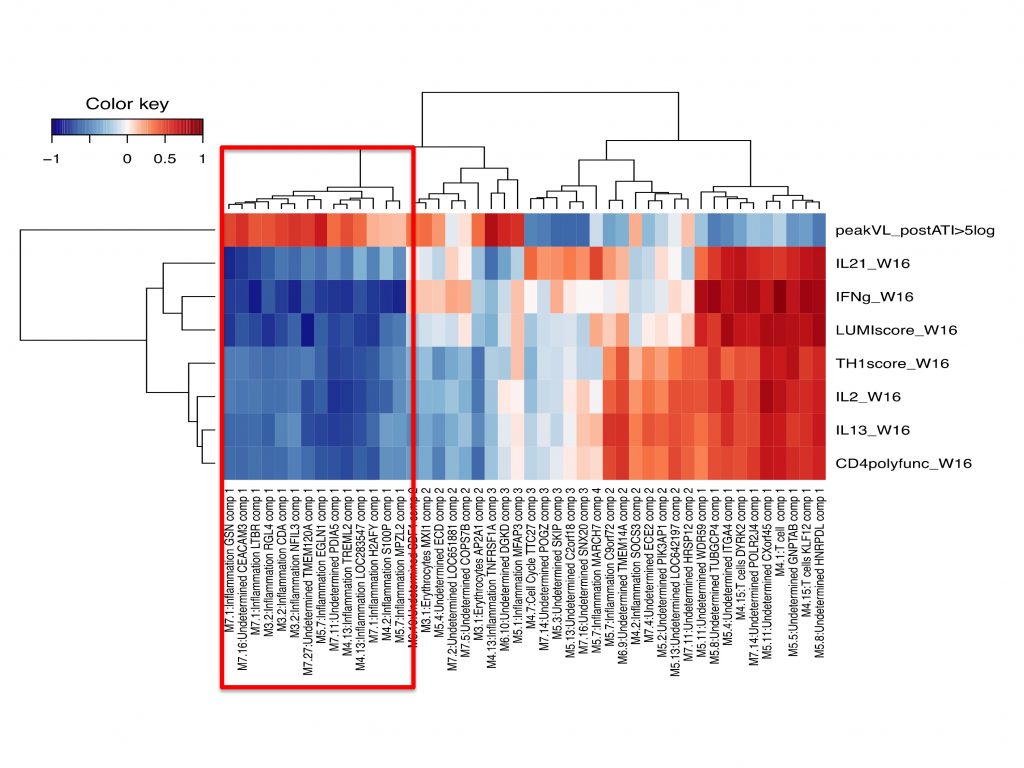
Correlations between vaccine-elicited immune responses and the magnitude of viral rebound post c-ART cessation in the anti HIV therapeutic trial DALIA (Transcriptomic analysis)
More specifically, the aims of the team are: i) to understand how and why HIV pathophysiology remains a major clinical problem despite the advent of several generations of potent antiviral drugs; ii) to develop innovative immune interventions such as cytokines, immunomodulators and vaccines aimed at preventing either HIV infection or persistent pathogenesis. Our strategy is to develop in vitro models, to exploit what we can learn from longitudinal follow up of large cohorts of patients at various stages of the disease and following immune interventions in preclinical or clinical studies. This strategy benefits also from a “systems biology” approach allowing a large exploration of hypotheses from limited amount of human materials (blood, sera, cells or gut and skin biopsies) and the development of new vaccines, the validation of which brings also new knowledge on the physiology and immune correlates of protection. Our path is to investigate mechanisms of immune restoration, the effects of immune modulation strategies, and the consequences of immune interventions.
Work program of the team includes three main aspects :
Immune restoration (PI:Pr JD Lelièvre)
This part of the program is aimed at pursuing the investigation of the mechanisms involving IL-7 and Notch in the generation of early thymic precursors but also aspect of regulation of T cell homeostasis during HIV infection. Currently topics developed here are 1. Interaction of Notch and IL7 in T cell development 2. Analysis of CD34 potential during HIV infection 3. Determination of the role of SamHD1 in peripheral T cell during HIV infection
Immune regulation (PI: Pr S Hue, Dr H Hocini, Dr C Lacabaratz, Pr JD Lelièvre)
A large part of our program aims at understanding the regulation of effector T cell responses with a specific focus on Treg in the periphery and at mucosal sites in HIV infected individuals, co-infected or not with other pathogens. The Current main topics developed are: 1. : Investigation of the interaction between Regulatory/effector balance and epithelial damage in HIV + individuals; 2. B cells response and impact on the gut integrity in HIV infected patients; 3. Study of the role of Treg in vaccination; 4. Study of Treg/Th17 balance during Mycobacterium Tuberculosis infection in patients co-infected or not with HIV
In collaboration with the clinical department of dermatology of CHU Henri Mondor (Pr O Chosidow, Pr P Wolkenstein) the team also perform research on hidradenitis suppurativa which is used as a model to of skin inflammation and T cell homeostasis disturbance.
Immune intervention (PI: Dr S Cardinaud, Pr V Godot, Dr H Hocini, Dr C Lacabaratz, Pr JD Lelièvre, Dr G Scarlatti,Pr S. Hue)
As a part of the VRI our team in collaboration with our clinical department is involved in a large clinical program aiming to develop prophylactic and therapeutic phase I/II HIV prime boost vaccine studies to identify the “best-in-class” strategy that will be developed in phase IIb/II. The vaccine strategy of the VRI focuses on dendritic cells using anti DC targeting vaccines. More specifically, the current topics developed are
1. The study of antibody responses at the mucosal level during HIV infection and vaccination;
2. Study of the role of GILZ protein in the biology of DC and its use to improve DC;
3. Study of the direct impact of Env-anti-DC proteins on both phenotype and function of DCs.
4. Building of prophylactic and therapeutic vaccine trials (clinical department)
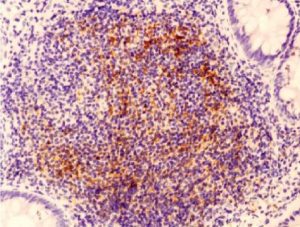
Histological aspect of the gut in an early treated patients showing a well-organized B-cell follicle containing a germinal center highlighted by a network of CD21+ FDC
Key words
HIV, vaccine, Treg, Dendritic cells, GILZ, IL7, CD34, Notch, Mucosal immunity, hidradenitis suppurativa